Clinical pipeline
Overview of Lipum’s Development Program
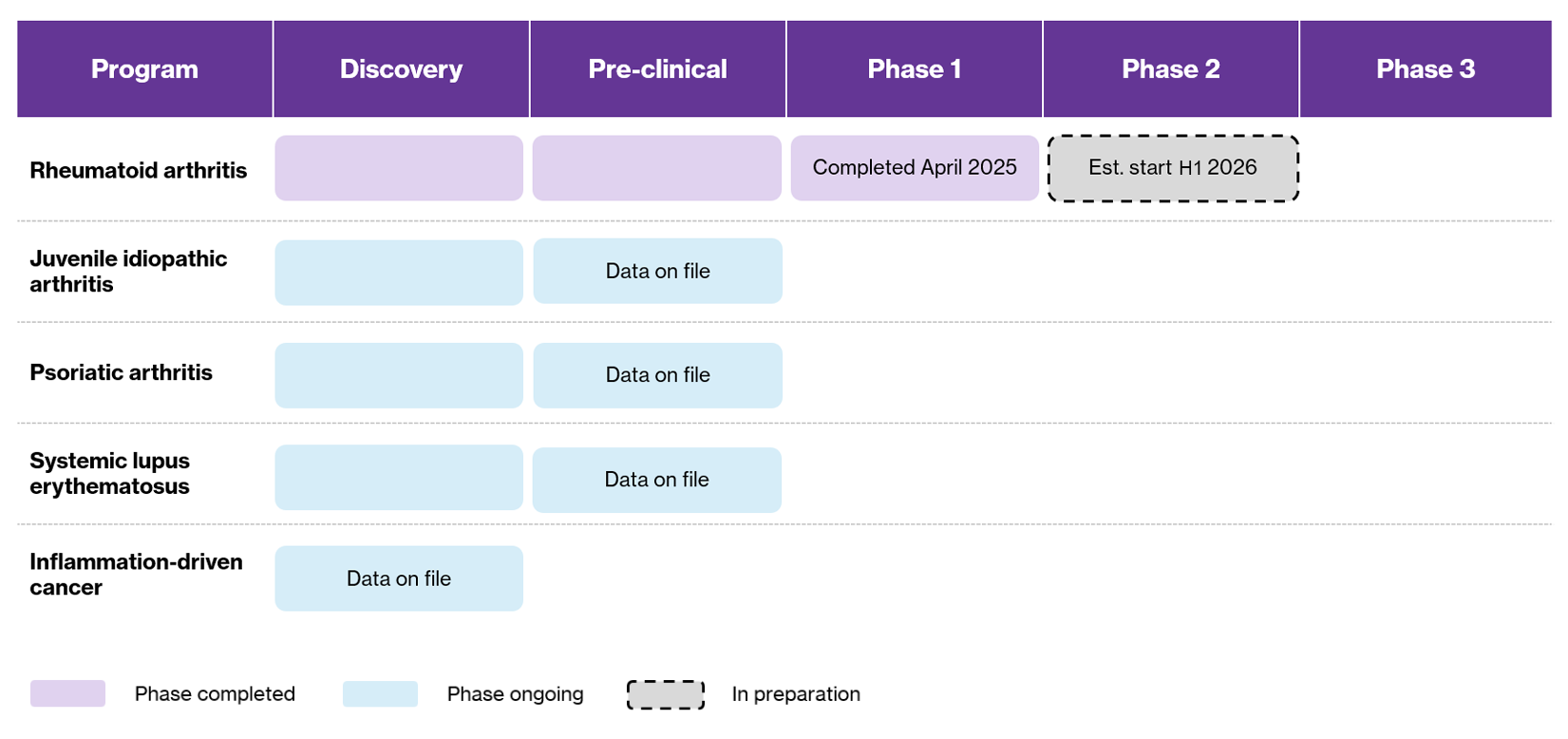
Lipum is advancing the development of SOL-116, a novel humanized antibody that targets bile salt-stimulated lipase (BSSL)—a unique target protein involved in the pathogenesis of several chronic inflammatory diseases. The company’s lead indication is rheumatoid arthritis (RA), with additional planned targets including juvenile idiopathic arthritis (JIA), systemic lupus erythematosus (SLE), psoriatic arthritis (PsA), and inflammation-driven cancers.
A significant milestone was reached in April 2025 with the completion of Lipum’s first human clinical trial: a Phase 1 First-in-Human (FIH) study. Building on encouraging results from the phase 1 study, Lipum is now preparing to initiate a Phase 2 clinical trial in RA, expected to begin in H1 2026. The study will focus on demonstrating clinical efficacy, refining the dosing strategy, and further exploring the drug’s mechanism of action in RA patients. Concurrently, the company is advancing preclinical and strategic preparations for trials in additional inflammatory conditions in which BSSL plays a key pathological role.
